Overview
Tirzepatide, a compound used for managing type 2 diabetes and promoting weight loss, may pose safety risks due to its lack of FDA oversight and documented adverse events. Therefore, thorough consultation with healthcare professionals before use is essential.
While tirzepatide can be beneficial in managing diabetes and aiding weight loss, the potential for serious side effects highlights the importance of tailored medical guidance. This underscores the need for cautious implementation, ensuring that individuals are well-informed and supported in their treatment decisions.
Introduction
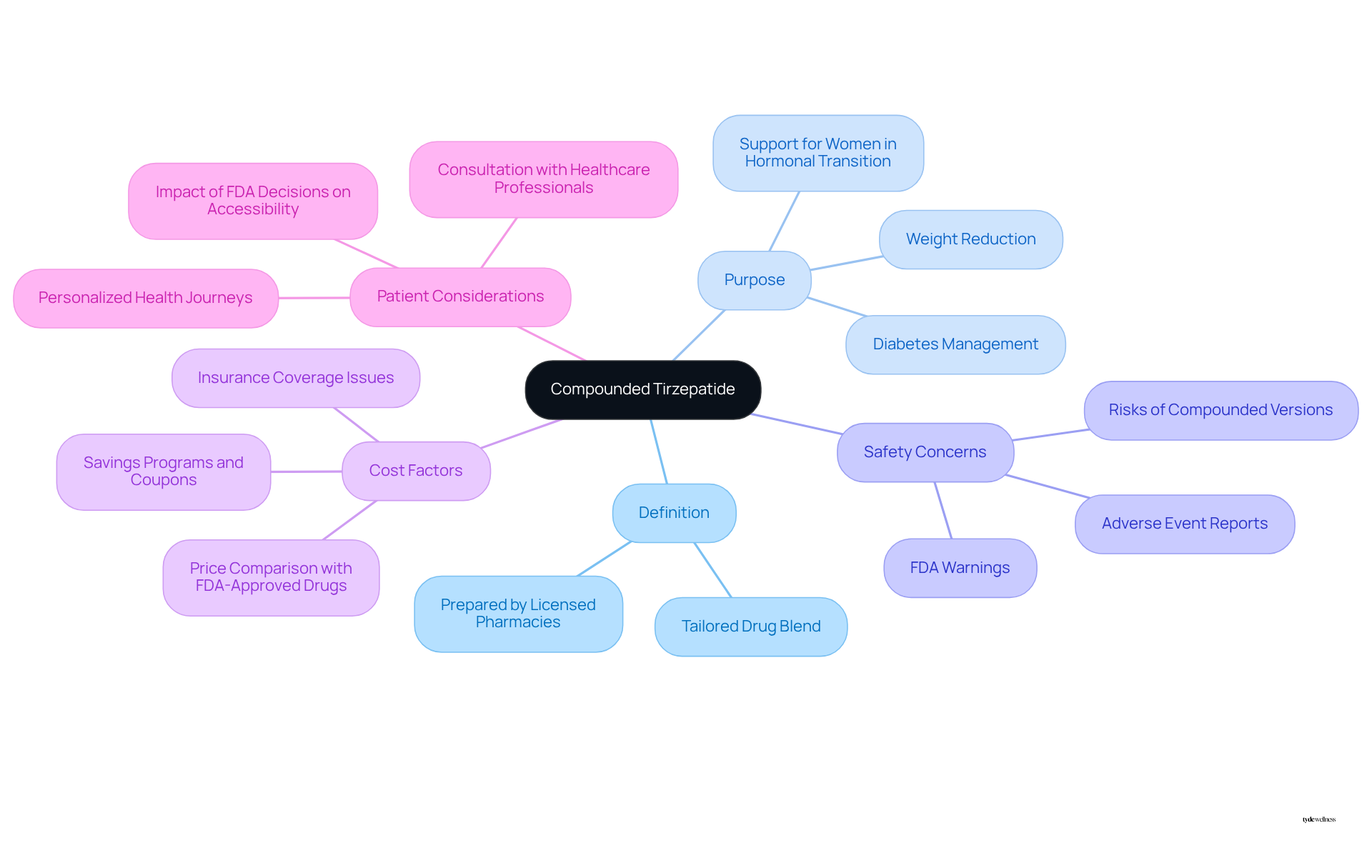
Compounded tirzepatide is a tailored formulation designed to assist in managing type 2 diabetes and facilitating weight loss. This treatment has emerged as a potential game changer for women experiencing hormonal shifts. However, significant concerns regarding its safety arise, particularly due to the lack of rigorous FDA oversight compared to commercially available alternatives. As reports of adverse events continue to surface, the pressing question remains: how can women navigate the complexities of compounded tirzepatide to ensure both its effectiveness and their safety?
Understand Compounded Tirzepatide: Definition and Purpose
Compounded formulation is a tailored blend of the drug, primarily utilized for managing type 2 diabetes and assisting in weight reduction. Unlike commercially available versions such as Mounjaro or Zepbound, customized tirzepatide is prepared by licensed pharmacies to meet specific patient needs. This treatment functions by imitating hormones that control appetite and glucose levels, rendering it a significant resource for women, particularly those undergoing hormonal shifts during perimenopause or menopause.
However, it is essential to acknowledge the FDA’s warnings concerning the risks linked to modified versions of FDA-approved medications, particularly when assessing if compound tirzepatide is safe, as they do not undergo the same safety, effectiveness, or quality reviews. Recent reports suggest that the FDA has obtained numerous adverse event notifications related to the specific formulation, which brings up the important question: is compound tirzepatide safe, emphasizing the necessity of consulting healthcare professionals prior to use. Furthermore, the FDA has announced a resolution to the shortage of Eli Lilly’s medication, which may influence the accessibility of customized versions.
Cost factors are also important, as the formulated medication may differ in price compared to FDA-approved alternatives. Understanding its definition and purpose helps women recognize its potential role in their health journey, particularly in achieving sustainable weight loss.
Evaluate Safety Risks: Potential Side Effects and Legal Concerns
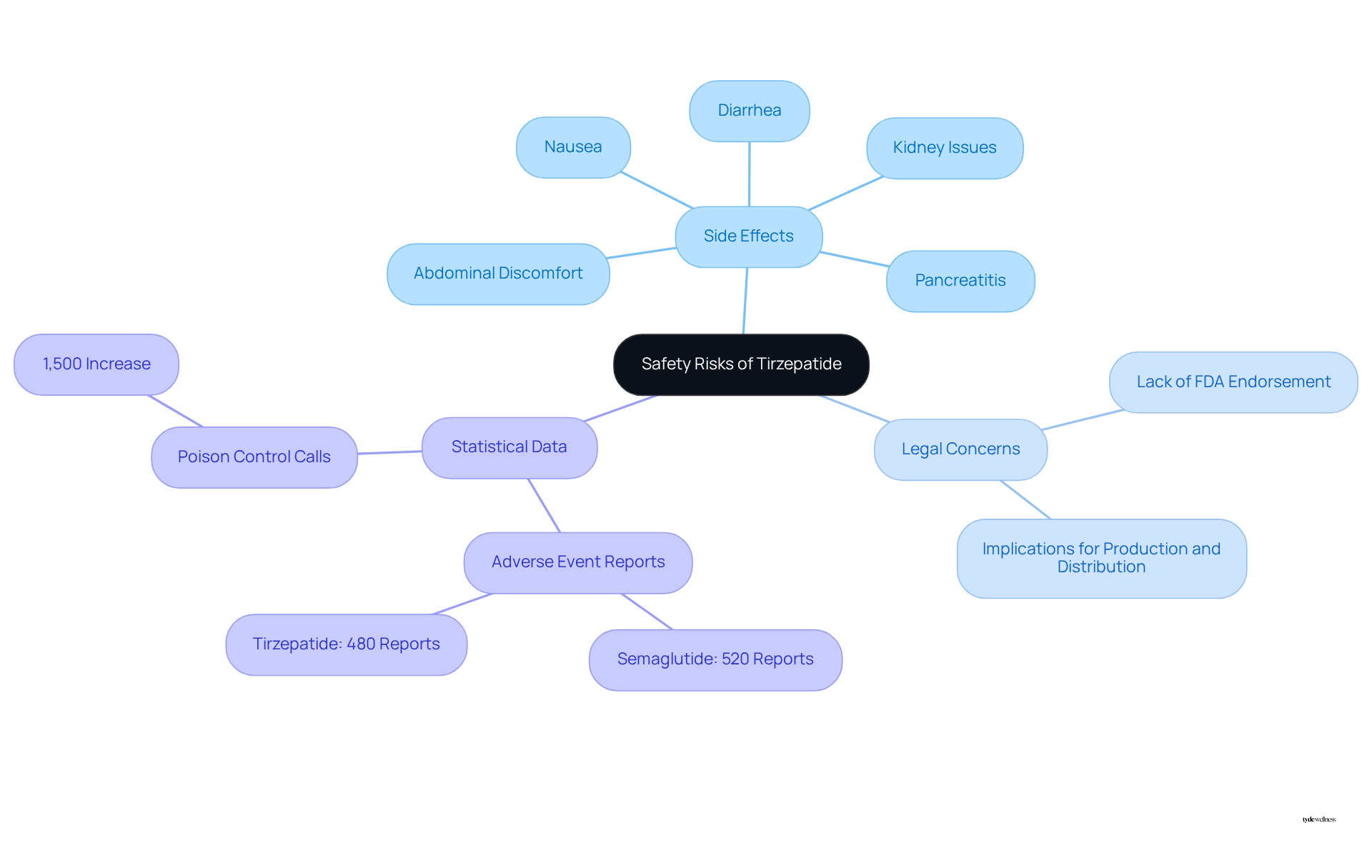
When assessing tirzepatide formulations, it is crucial to evaluate if the compound tirzepatide is safe. Typical side effects include nausea, diarrhea, and abdominal discomfort, which often occur as the body adapts to the treatment. More serious complications, such as pancreatitis and kidney issues, have also been documented. Significantly, mixed treatments lack FDA endorsement, raising serious legal issues regarding their production and distribution procedures.
The FDA has received 520 reports of adverse events linked to semaglutide preparations and 480 reports linked to tirzepatide preparations, highlighting the risks involved. Furthermore, there has been a nearly 1,500% increase in calls to poison control centers related to accidental overdosing on injected weight-loss drugs, underscoring the urgency of these safety concerns. Research indicates that women may experience a higher incidence of gastrointestinal side effects compared to men, making it essential for them to approach these treatments with caution.
It is essential to discuss these risks with a medical professional to guarantee that the compound tirzepatide is safe and to manage the intricacies related to mixed drugs. As Jack Mountain, PharmD, mentions, ‘Ultimately, deciding whether to use a customized GLP-1RA is a personal choice that should involve a conversation between the patient and their prescriber.
Implement Safe Practices: Consult Healthcare Providers and Follow Dosing Guidelines
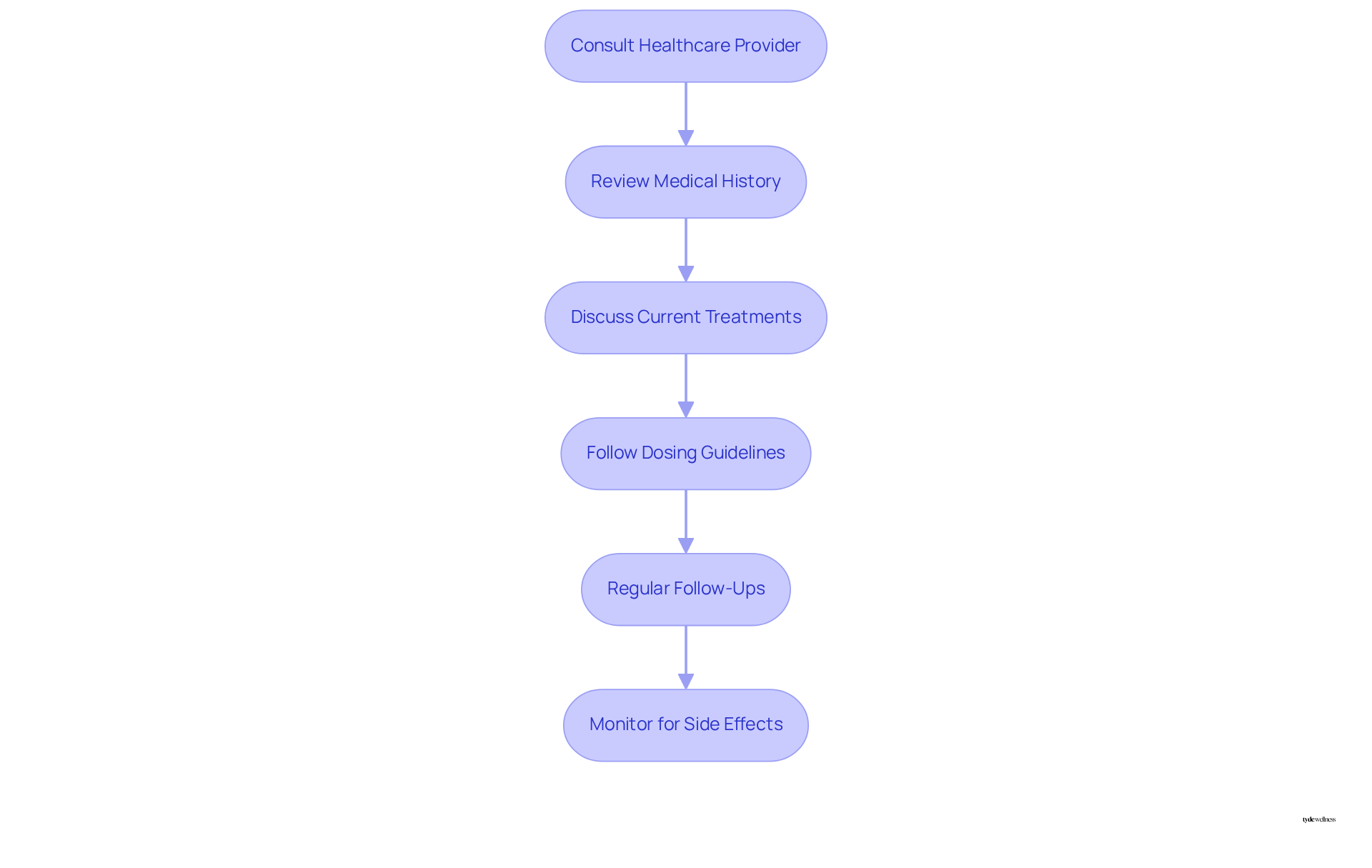
To ensure that compound tirzepatide is safe, it is crucial for women to prioritize consultations with healthcare providers who possess expertise in this drug and its effects. This initial discussion should include a comprehensive review of medical history, current treatments, and individual health objectives. Notably, approximately 12% of US adults have utilized a GLP-1 drug, highlighting the prevalence of such treatments.
Adhering to prescribed dosing guidelines is vital; treatment typically begins with a low dose, which can be incrementally adjusted based on tolerance and effectiveness. Regular follow-ups with the medical provider are essential for tracking progress and making necessary adjustments to the treatment plan.
Furthermore, it is important to recognize that customized medications do not undergo the same safety reviews as FDA-approved drugs, which leads to concerns about whether compound tirzepatide is safe, underscoring the necessity of consulting healthcare providers. Additionally, there have been reports of adverse occurrences associated with mixed formulations of the medication, emphasizing the importance of diligent oversight and compliance with protocols.
This proactive approach not only assists women in safely benefiting from the treatment but also reduces the risk of potential side effects, thereby promoting a more effective weight loss journey.
Monitor Progress: Regular Check-Ins and Adjustments for Optimal Results
Regular monitoring serves as a fundamental component in the effective use of compounded tirzepatide. Women are encouraged to schedule check-ins with their medical provider to discuss their experiences, including any side effects and weight changes. Additionally, maintaining a journal to track daily symptoms, dietary habits, and physical activity can yield valuable insights during these consultations. Based on the feedback from these interactions, healthcare providers can make necessary adjustments to the dosage or treatment plan, optimizing results. This ongoing evaluation not only aids in achieving weight loss goals but also addresses the question of whether compound tirzepatide is safe and effective over time.
![]()
Conclusion
Understanding the safety and effectiveness of compounded tirzepatide is crucial for women considering this treatment for managing type 2 diabetes and weight loss. Customized formulations offer a tailored approach to individual health needs; however, the lack of FDA oversight raises significant concerns about their safety and potential side effects. Engaging in thorough discussions with healthcare providers and adhering to prescribed guidelines is essential for navigating these complexities safely.
Key insights throughout the article highlight the necessity of evaluating the safety risks associated with compounded tirzepatide, including common side effects like nausea and more severe complications such as pancreatitis. The importance of regular consultations with medical professionals cannot be overstated, as these interactions facilitate necessary adjustments to treatment and ensure that women can safely benefit from this medication. Furthermore, ongoing monitoring of progress through check-ins and personal tracking can optimize results and address any emerging concerns.
In light of the potential risks associated with compounded tirzepatide, it is vital for women to prioritize their health by seeking professional guidance and making informed decisions. This proactive approach not only enhances the likelihood of achieving weight loss goals but also reinforces the significance of safety in the pursuit of effective treatment options. By staying informed and engaged in their health journey, women can navigate the complexities of compounded medications and make choices that support their well-being.
Frequently Asked Questions
What is compounded tirzepatide?
Compounded tirzepatide is a tailored blend of the drug primarily used for managing type 2 diabetes and assisting in weight reduction. It is prepared by licensed pharmacies to meet specific patient needs, unlike commercially available versions.
How does compounded tirzepatide work?
Compounded tirzepatide functions by mimicking hormones that regulate appetite and glucose levels, making it particularly beneficial for women experiencing hormonal changes during perimenopause or menopause.
What are the risks associated with compounded tirzepatide?
The FDA has issued warnings regarding the risks linked to modified versions of FDA-approved medications, including compounded tirzepatide, as they do not undergo the same safety, effectiveness, or quality reviews. There have also been reports of adverse events related to this formulation.
Should I consult a healthcare professional before using compounded tirzepatide?
Yes, it is essential to consult healthcare professionals prior to using compounded tirzepatide to assess its safety and suitability for your specific health needs.
What impact does the FDA’s resolution on Eli Lilly’s medication have on compounded tirzepatide?
The FDA’s resolution to the shortage of Eli Lilly’s medication may affect the accessibility of customized versions of tirzepatide, including compounded formulations.
How does the cost of compounded tirzepatide compare to FDA-approved alternatives?
The cost of compounded tirzepatide may differ from that of FDA-approved alternatives, and it is important to consider this when evaluating treatment options.
Who can benefit from compounded tirzepatide?
Women, particularly those undergoing hormonal shifts during perimenopause or menopause, may find compounded tirzepatide beneficial in managing their health and achieving sustainable weight loss.
List of Sources
- Understand Compounded Tirzepatide: Definition and Purpose
- FDA’s Concerns with Unapproved GLP-1 Drugs Used for Weight Loss (https://fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fdas-concerns-unapproved-glp-1-drugs-used-weight-loss)
- As Eli Lilly, compounders battle over weight loss drugs, patients are caught in the middle (https://statnews.com/2024/10/11/eli-lilly-tirzepatide-zepbound-mounjaro-compounded-drugs)
- Compound versions of GLP-1 drugs for weight loss halted by FDA (https://abcnews.go.com/GMA/Wellness/compound-versions-weight-loss-drugs-longer-fda-rules/story?id=119665010)
- Compounding pharmacies can resume making tirzepatide as FDA reconsiders shortage (https://nbcnews.com/health/health-news/compounding-pharmacies-can-resume-making-tirzepatide-fda-reconsiders-s-rcna174551)
- Evaluate Safety Risks: Potential Side Effects and Legal Concerns
- Compounded Weight Loss Medications: What are They and What are Their Risks? | Brown University Health (https://brownhealth.org/be-well/compounded-weight-loss-medications-what-are-they-and-what-are-their-risks)
- Concerns Surrounding Compounded GLP-1s Mount As Shortages of Tirzepatide, Semaglutide End (https://pharmacytimes.com/view/concerns-surrounding-compounded-glp-1s-mount-as-shortages-of-tirzepatide-semaglutide-end)
- Compounded Weight Loss Drugs Are Popular. But Are These Ozempic-Like Copies Safe? (https://news.cuanschutz.edu/news-stories/compounded-weight-loss-drugs-are-popular.-but-are-they-safe)
- FDA’s Concerns with Unapproved GLP-1 Drugs Used for Weight Loss (https://fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fdas-concerns-unapproved-glp-1-drugs-used-weight-loss)
- Are Compounded GLP-1 Drugs Safe? (https://jamanetwork.com/journals/jama/fullarticle/2829690)
- Implement Safe Practices: Consult Healthcare Providers and Follow Dosing Guidelines
- Compounded Weight Loss Drugs Are Popular. But Are These Ozempic-Like Copies Safe? (https://news.cuanschutz.edu/news-stories/compounded-weight-loss-drugs-are-popular.-but-are-they-safe)
- FDA’s Concerns with Unapproved GLP-1 Drugs Used for Weight Loss (https://fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fdas-concerns-unapproved-glp-1-drugs-used-weight-loss)
- Are Compounded GLP-1 Drugs Safe? (https://jamanetwork.com/journals/jama/fullarticle/2829690)
- Are Compounded GLP-1 Weight Loss Drugs Safe and Legal? (https://everydayhealth.com/weight/is-it-safe-to-use-compounded-semaglutide-and-tirzepatide-for-weight-loss)
- Monitor Progress: Regular Check-Ins and Adjustments for Optimal Results
- Tirzepatide Weight Loss Success Stories: Real Results You Need to See (https://healthon.com/blogs/journal/tirzepatide-weight-loss-success-stories-real-transformations?srsltid=AfmBOopGL2LcQU1PAlPB-XWE-t3ah-jKAR5yxvt7vhG1AnW8aNJ7c4NZ)
- Tirzepatide (subcutaneous route) – Side effects & dosage (https://mayoclinic.org/drugs-supplements/tirzepatide-subcutaneous-route/description/drg-20534045)
- Tirzepatide (Mounjaro®) for the management of obesity (https://woodlandshealthcentre.org.uk/news/tirzepatide-mounjaro)
- How to Maximize Results from Tirzepatide? (https://getheally.com/patients/news/how-to-maximize-results-from-tirzepatide)
- Are the New Weight Loss Drugs Too Good to Be True? (https://magazine.ucsf.edu/weight-loss-drugs-too-good-to-be-true)



